Measurement of induced chlorophyll fluorescence
Method principle
This method is based on a knowledge achieved in the 1930s by Kautsky and Hirsch (1934), that photosynthetic organisms containing chlorophyll a emit out some portion of captured light energy in a form of fluorescent radiation in visible part of the spectrum (especially its red to near infra-red area around 685 nm and 735 nm). Later this phenomenon was explained as a part of mechanisms for dissipation of excessive energy derived from captured photons.
Chlorophyll fluorescence represents solely a physical event, but changes in the intensity of fluorescence emission reflect the course of photochemical reactions in primary (so called “light”) phase of photosynthesis: photon capture, excitation of chlorophyll a molecules by their energy and a release of energy-rich electrons, from which the energy is transferred into chemical bonds of ATP and NADPH2 for later utilization.
The fluorescence emission is induced by short weak pulses of measuring light and is recorded by a sensitive detector. The detector is equipped by long pass wavelength filter and therefore only induced blinks of fluorescence radiation can pass through. Due to a dynamics of changes in fluorescence intensity, recorded in some time scale, it is possible to describe changes on biochemical level in photosynthetic apparatus of plant, algae or cyanobacteria, and to consider roughly the efficiency of photosynthesis in studied organism. Photosynthetic apparatus of photo-autotrophic organisms is highly sensitive to environmental changes caused by both physical (e.g. temperature, excessive irradiation, UV-light, drought etc.) and chemical factors (the presence of toxic organic compounds both natural and artificial like herbicides, the effect of heavy metals, ozone and another compounds, including the deficiency of nutrients).
Advantage of this method is, that the measurement could be done quickly, non-destructively and repeatedly both in field and in the lab. It is successfully used in plant stress physiology in studies on the effects of above mentioned factors. One disadvantage is, that various kinds of stress can evoke similar non-specific response and thus the possibility to use fluorescence as a direct proof of an effect of specific factor is limited, especially when co-operation of several factors is possible.
Induced chlorophyll fluorescence at CCT
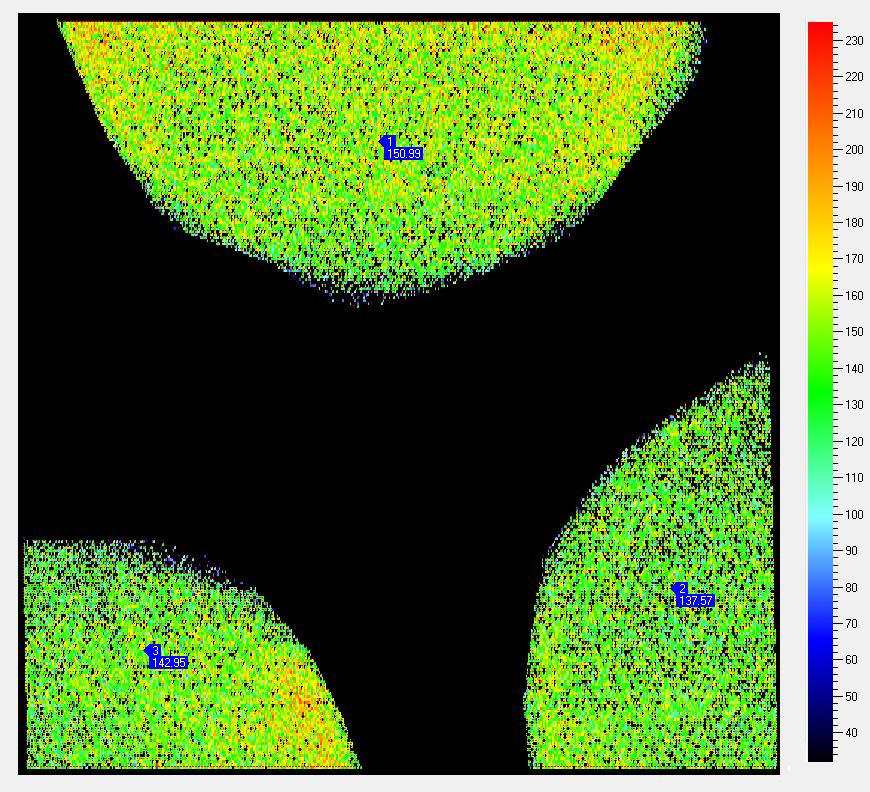
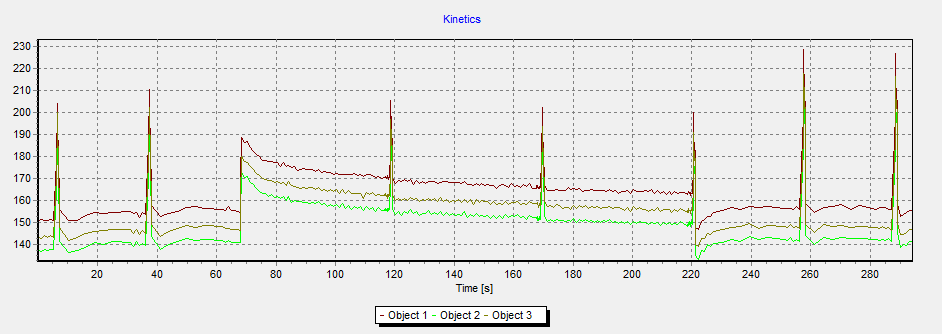
In our lab we use fluorescence cameras FluorCam MF700 developed by Photon System Instruments in two different versions – a stand-alone camera with shielded measuring chamber for macro objects and a camera connected to a microscope for studying of native microsamples. Built-in sources of measuring, actinic and saturating light in combination with analysis software (FluorCam v.5 and v.6) enable to used them as PAM fluorometers (Pulse Amplitude Modulation; e.g. Rohacek and Bartak 1999). By means of them we can evaluate a series of fluorescence parameters derived from kinetics received during Kautsky effect measurements (irradiation of a dark-adapted sample by continuous light) or changes in the participation of photochemical and non-photochemical utilization of excitation energy by a method of saturation pulses (except measuring and continuous actinic light the sample is exposed to short pulses of very strong light that causes time-limited reversible overloading of photosynthetic apparatus by excitation energy and allows to evaluate its capability to transfer the energy).
Both cameras are commonly used for rapid evaluation of photosynthetic activity of both natural and laboratory suspensions of phytoplankton as a supplemental method in experiments conducted at our department.
Kautsky, H., Hirsch, A. 1934. Chlorophyllfluoreszenz und Kohlensäureassimilation. Das Fluoreszenzverhalten grüner Pflanzen. Biochem. Zeitschrift 274: 423-434.
Rohacek, K., Bartak, M. 1999: Technique of the modulated chlorophyll fluorescence: basic concepts, useful parameters, and some applications. Photosynthetica 37(3): 339-363.